- Went over our homework
- We did a lab based on 'polarity'
- Before that, however, we did some notes:
Solvents and Solutes can be Polar or Non-polar
Non-polar substances have equal charge distribution (symmetrical)
Polar substances have an unequal charge distribution (asymmetrical)
H2O = polar:
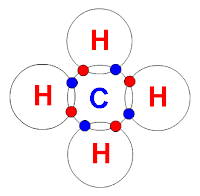
CH4 = nonpolar:
_________________________________________________________
The lab's objective was to determine if Glycerin is polar or non-polar.
Here's the background information that was written on the lab:
- Sodium chloride is an ionic solid crystalk that forms a crystal lattice structure. When dissolved in solvents, this lattice breaks up and the ions dissociate.
- Sucrose is table sugar and like Sodium Chloride it also forms a crystal structure. Unlike Sodium Chloride however, Sucrose is not ionic; it is molecular. The structure of Sucrose makes the molecule polar.
- Iodine, like Sucrose, is molecular and also forms crystals. However the crystals of Iondine are non-polar.
- Water is a polar solvent
- Paint thinner is a non-polar solvent
- Glycerine is a polar solvent
The materials we needed were test tubes, test tube stoppers, a test tube rack, scupula, safety goggles and an apron, sodium chloride, sucrose, iodine crystals, paint thinner (Turpentine) and Glycerin
The entire procedure basically involved us seeing whether or not the solutes (like table salt, sugar and iodine) dissolved in the solvents (water and paint thinner). We learned that polar solutes dissolve in polar substances and non-polar solutes dissolved in non-polar substances (LIKE DISSOLVES LIKE)




No comments:
Post a Comment